Research within our group
Switchable Molecules for Biomedical Applications
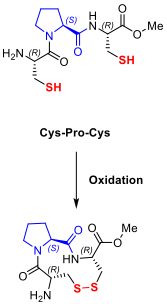
- Switchable peptides
- Proline-rich peptides
- Oxidative folding of cysteine-rich peptides
- Ion-peptide interactions
- Switching via external triggers
Ionic Liquids
- Task-specific ionic liquids
- Ionic interactions
- NMR in neat ionic liquids
Switchable Solvents
- Switchable polarity solvents
- Switchable hydrophilicity solvents
New Chiral Anions
- Chiral ionic liquids
- Strong chiral Brønstedt acids